- News
24 April 2012
Magnesium puzzle solving in nitride semiconductors
University of California Santa Barbara (UCSB) scientists have reported density functional calculations that shed light on the puzzling behavior of magnesium (Mg) as p-type (acceptor) dopant in nitride semiconductor materials involving the elements gallium (Ga), aluminum (Al) and indium (In) [John L. Lyons et al, Phys. Rev. Lett., vol108, p156403, 2012].
In particular, they explain some puzzles about the behavior of photoluminescence (PL) spectra, before and after annealing. Such spectra are important characterization tools, and correct identification of the sources of spectral lines should lead to improved technological development and production processes.
The development of Mg-doping in gallium nitride (GaN) during the 1980s was one of the most important ingredients leading to the present applications of nitride semiconductors in visible and ultraviolet light-emitting devices.
In GaN, the Mg atoms tend to substitute for the Ga in the crystal structure (symbolized as MgGa). The addition of Mg in the crystal structure is known to create conditions where electrons can be grabbed from the valence band, leaving holes. Unfortunately, the activation energy for this (~200meV) is quite high when compared with typical thermal excitations at room temperature (~26meV). By contrast, silicon doping creates n-type behavior in GaN much more easily, donating electrons to the conduction band with activation energies around 20meV.
In photoluminescence experiments, there are two extra peaks in Mg-doped GaN: one is an ultraviolet emission with a photon energy of 3.27eV; the other is a blue peak of energy 2.8eV. Naively, one might attribute the 3.27eV emission to electrons from the conduction band falling into the Mg-state near the valence band. If Mg behaves as a shallow acceptor, then this emission should indeed occur at an energy close to the 3.5eV bandgap. In this model, the blue emission would be associated with (unidentified) compensating donors induced by Mg.
However, the UV signal is reduced when such samples are annealed to improve their p-type properties, which would suggest that the peak is not associated with Mg itself, but with Mg-H complexes, since the annealing is expected to drive off hydrogen (H) from the sample. The blue emission would then be related to the acceptor mechanism of Mg proper. However, if Mg is an acceptor with activation energy around 200meV (0.2eV), which is relatively close to valence band maximum, how can blue light be emitted from transitions from the conduction band?
In their calculations, the researchers find that the bound hole (Mg0Ga) is highly localized, with most of its charge on the nearest-neighbor axial nitrogen (Figure 1a). Normal shallow acceptor behavior consists of a delocalized hole only loosely bound to the negative charge acceptor core.
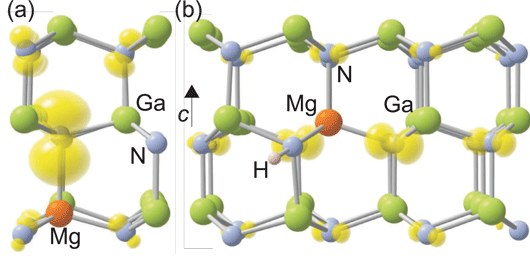
Figure 1: (a) Local structure showing distortion around neutral Mg acceptor in GaN. Localization of hole on axial nitrogen neighbor is illustrated by spin-density isosurface (yellow), set to 5% of maximum. (b) Structure of Mg-H complex in GaN in positive charge state. The spin density shows the more delocalized nature of the complex. Large green spheres denote Ga atoms, medium light blue spheres denote N atoms, and orange (darker) sphere denotes MgGa.
In addition to the hole charge being localized on the neighboring nitrogen atom, the crystal lattice is distorted by a 15% lengthening of the bond between the Mg and axial N atoms, compared with the bulk Ga-N bond. The configuration where the hole is localized on the planar N atoms has an energy level that is 0.03eV higher. The researchers add: “Careful investigations reveal that there are no other metastable configurations, in contrast to previous theoretical work.”
The localized nature of the hole in the bound state suggests to the researchers that its use as a shallow acceptor is an accident of nature. When the hole is freed (Mg-Ga), the lattice is no longer asymmetrically distorted and only ‘breathing relaxations’ of the nearest neighbors occur to accommodate the Mg in the crystal.
The researchers explain the blue transition as being due to recombination of an electron with an Mg0Ga state, creating an Mg-Ga state. However, the optical transition tends to result in a state that is initially asymmetrically distorted, and so has higher energy than the Mg-Ga state at 260meV above the valence-band maximum referred to above.
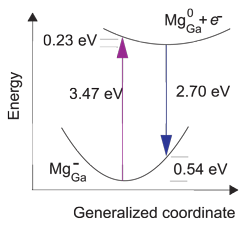 Representing the distortion asymmetry by a generalized coordinate, the researchers give an energy diagram (Figure 2b) with an emission peak around 2.70eV. The energy for relaxation to the symmetric breathing state is about 0.54eV. The large value of 0.54eV indicates that the photoluminescence should be broad, as seen experimentally.
Representing the distortion asymmetry by a generalized coordinate, the researchers give an energy diagram (Figure 2b) with an emission peak around 2.70eV. The energy for relaxation to the symmetric breathing state is about 0.54eV. The large value of 0.54eV indicates that the photoluminescence should be broad, as seen experimentally.
Figure 2: Configuration-coordinate diagram illustrating optical processes related to Mg in GaN. Recombination of an electron at conduction-band minimum with Mg0Ga leads to emission peaking at 2.70eV, explaining the blue line emission in GaN.
The researchers also considered the way in which interstitial hydrogen atoms affect the Mg acceptors in GaN. They find that the H+ forms a neutral bound state with Mg-Ga, with a binding energy of 1.02eV. Also, when the Fermi level is close to the valence-band maximum, there is a stable positively charged state 130meV above the valence-band maximum (Figure 1b).
One effect of the H-interstitial is to reduce the lattice distortion. In particular, the distinct axial character of the distortion disappears. The positive charge state also gives a route for an ultraviolet transition from the conduction band, with a predicted peak around 3.32eV. The researchers would associate this with the experimental ultraviolet emission around 3.27eV.
The electrical effect is two-fold. The neutral state inactivates the Mg-acceptor; the positive state is a compensating center that removes a hole from the valence band. The researchers comment: “Thus, these complexes are doubly detrimental in reducing free hole concentrations in Mg-doped GaN. Combined optical and electrical experiments to assess the presence and role of these complexes in Mg-doped GaN are called for.”
The ultraviolet line is especially strong in samples that have not been annealed. “Confirmation of our proposed attribution could be obtained by monitoring the intensity of the UV line in material that is first activated and then intentionally hydrogenated: the formation of Mg-H complexes should lead to an increase in the intensity of the UV line,” the researchers add.
The researchers made similar calculations with InN and AlN. In AlN, in addition to the negative and neutral states, a positively charge state (Mg+Al) forms that is stable when the Fermi level falls below 0.36eV, relative to the valence-band maximum. In addition to a greater axial distortion of 18%, the positive state indicates that it is more difficult to exhibit p-type conductivity in Mg-doped AlN (and by implication AlGaN). The poor conductivity of p-AlGaN/p-AlN is already well known experimentally and theoretically.
By contrast with GaN and AlN, the researchers find genuine shallow acceptor behavior with Mg-doped InN. There are no asymmetric distortion or hole localization effects. The researchers give an energy transition for transitions between the Mg0In and Mg-In states as 190meV, consistent with experimental values.
The researchers believe that the behavior of Mg as an acceptor in nitride semiconductors is linked to the character of the valence band. Deep, flat bands (with implied higher hole effective mass) promote hole localization on the acceptor. AlN has the largest bandgap (~6.15eV in the UCSB calculation), the deepest valence band and highest hole effective mass of the nitride semiconductors, leading to the highest acceptor ionization energy. InN has the smallest bandgap of the nitride semiconductors (~0.65eV, according to calculation), with a high valence band and smaller hole effective mass.
The researchers comment: “Our results resolve experimental results for GaN that have remained puzzling for almost two decades: MgGa is responsible for the widely observed blue luminescence, and the Mg-H complex is responsible for 3.27eV PL. MgIn in InN is found to behave as a shallow, effective-mass acceptor, with the lowest ionization energy among the nitrides.”
Magnesium p-type dopant nitride semiconductors GaN
http://prl.aps.org/abstract/PRL/v108/i15/e156403
The author Mike Cooke is a freelance technology journalist who has worked in the semiconductor and advanced technology sectors since 1997.
