- News
16 March 2011
Probing vacuum-deposited copper-zinc-tin-chalcogenide kesterite anneal process
University of Luxembourg researchers have been studying the feasibility of creating low-cost, manufacturable copper-zinc-tin-chalcogenide kesterite-based solar cells using vacuum processing [Alex Redinger et al, J. Am. Chem. Soc., vol133, p3320, 2011]. The team found that introducing tin (Sn) into the post-deposition annealing box prevented decomposition of copper-zinc-tin sulfide (Cu2ZnSnS4) and selenide (Cu2ZnSnSe4) materials (CZTS(e)). High-temperature annealing is required to control film composition and homogeneity.
Last year, a record efficiency of 9.7% was achieved by an IBM team using a liquid deposition process of such materials. The constituents were dissolved in hydrazine (N2H4) solution. The deposition was onto molybdenum-coated glass, followed by a short anneal at 540°C. The IBM process has since been the subject of joint development agreements with Solar Frontier of Japan (Japan’s Solar Frontier to co-develop IBM’s CZTS solar cell technology ) and DelSolar of Taiwan (DelSolar and IBM to jointly develop solar cell technology).
The main attraction of these materials is the use of high-abundance, low-cost elements (Figure 1., e.g. no indium). The resulting semiconductor layers have a tunable bandgap in the range 1–1.5eV that is suitable for creating solar cells.
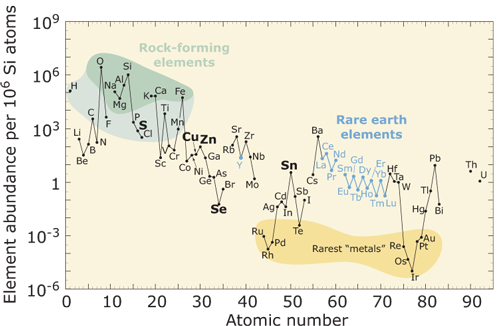
Figure 1: Relative abundance (atom fraction) of the chemical elements in Earth’s upper continental crust as a function of atomic number. Of course, deposits exist where the proportions are much different from these averages, making for economic extraction. From http://pubs.usgs.gov.
Although a liquid-based process has its attractions in terms of low production costs, the use of hydrazine solution is a safety concern. Hydrazine is a highly toxic and dangerously unstable compound, particularly when out of water. The instability of hydrazine has been used to make rocket fuels, first with German rocket planes in World War II.
The vacuum process alternative to liquid CZTS(e) deposition has achieved efficiencies up to 6.8%. Normally, one would expect a vacuum process to result in better-quality material (e.g. less defects/dislocations) and hence enhanced solar cell performance. Also, vacuum processes are attractive for depositing a wide range of other materials with high quality.
The researchers explain the failure to deliver better-quality CZTS(e) cells by saying that the annealing step used to improve the crystal structure after deposition results in loss of tin from the vacuum-deposited CZTS semiconductor material, reducing conversion efficiency.
The Luxembourg team produced solar cells annealed in an excess sulfur atmosphere (process A) or in sulfur/tin (process B). The process A annealing atmosphere was created by using sulfur pellets and forming gas (hydrogen/nitrogen). Process B was achieved by also placing 1mg of tin in the graphite annealing box. The 2-hour annealing was carried out at 560°C.
Without tin being present during annealing, the resulting solar cell had a conversion efficiency of 0.02%, and poor short-circuit current and open-circuit voltage characteristics (0.72mA/cm2 and 80mV, respectively).
The effect of having tin in the annealing box was to boost efficiency to 5.4%. The short-circuit current and open-circuit voltage were 20mA/cm2 and 497mV, respectively. The researchers have previously produced a 3.2%-efficient cell with just sulfur annealing; they therefore believe that the result of process B can be further optimized.
Since the publication of these results, Luxembourg’s kesterite solar cells have improved to 6.1% efficiency, certified by Fraunhofer ISE as a European record.
The researchers understood their results in terms of the decomposition reaction:
Cu2ZnSnS4(solid) Û Cu2S(solid) + ZnS(solid) + SnS(gas) + (1/2)S2(gas) (equation 1)
Although the presence of sulfur in the anneal box might be thought sufficient to block the decomposition reaction to the right-hand side of equation 1, the partial pressure needed is high. This is because most of the elemental sulfur in the annealing atmosphere comes in the form of sulfur rings of eight atoms, rather than the S2 needed. The presence of tin in the box leads to SnS on heating, which is much more effective in blocking decomposition.
The researchers further studied the decomposition process by first electro-depositing copper and zinc onto molybdenum-coated glass. The thickness of the Cu/Zn layers can be used to control the composition of the final CZTS material.
The team then performed three anneals in sequence in various atmospheres. Between each stage a series of material analyses were carried out (grazing incidence x-ray diffraction, energy- and wavelength-dispersive XRD). The first anneal was in sulfur, the second with a SnS/S atmosphere, and the third in vacuum. All anneals were at 560°C. The duration of the first two anneals was two hours; the final vacuum anneal was six hours.
Analysis (Figure 2) of the first anneal revealed the presence of grains ZnS and Cu9S5 (copper-poor Cu2S). The two materials do not intermix.
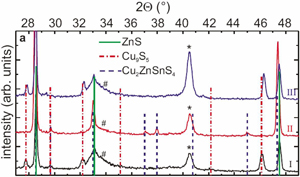 Figure 2: Grazing incidence x-ray diffraction pattern of Luxembourg’s annealing experiments, elucidating the equilibrium proposed in equation 1. Scan (I): after annealing of a Cu/Zn stack in sulfur. Scan (II): after annealing of a mixed Cu9S5 and ZnS film in sulfur and SnS(g). Scan (III): after annealing CZTS in vacuum. Identified phases include S, Cu9S5, Cu2ZnSnS4, Mo substrate (*) and MoS2 (#).
Figure 2: Grazing incidence x-ray diffraction pattern of Luxembourg’s annealing experiments, elucidating the equilibrium proposed in equation 1. Scan (I): after annealing of a Cu/Zn stack in sulfur. Scan (II): after annealing of a mixed Cu9S5 and ZnS film in sulfur and SnS(g). Scan (III): after annealing CZTS in vacuum. Identified phases include S, Cu9S5, Cu2ZnSnS4, Mo substrate (*) and MoS2 (#).
The second anneal created CZTS and the CuxS signal disappears. Unfortunately it is not possible to separate the ZnS and CZTS signals to say whether there is any remaining material with the former composition. However, the copper, zinc and tin are mixed near the optimum ratios needed for high-performance solar cells.
The researchers comment: “This shows that the incorporation of Sn via SnS does not proceed in a random way but is self-limiting. As long as enough CuxS and ZnS is present, SnS and S from the gas phase are incorporated in the film to form CZTS.”
Experiments were also carried out where the annealing was carried out with a Se/SnSe2 atmosphere, which also showed a self-limiting behavior. One must remember here that selenium is not a particularly common element (Figure 1).
The researchers comment that their work “can be used to simplify the four-dimensional parameter space (spanned by the four different elements) to an easy and robust two-dimensional process”. They add that their study of the decomposition reaction “enables us to simplify the precursor to a film containing only Cu and Zn, whereas Sn and S(e) are introduced from the gas phase by a self-regulating process.”
DOE’s PV Incubator program invests $7m in fourth round of projects
Japan’s Solar Frontier to co-develop IBM’s CZTS solar cell technology
DelSolar and IBM to jointly develop solar cell technology
The author Mike Cooke is a freelance technology journalist who has worked in the semiconductor and advanced technology sectors since 1997.
Join Semiconductor Today's LinkedIn networking and discussion group
